pH soil
The pH amount as the primary indicator for the biochemical activity in soil, plays a basic role in diagnostic about growth or degradation of plant life. Agronomists and fans are encouraged to more and better investigate the links between biochemistry of the vegetable colture and the electrochemical potential measured by the pH meter in the soil, thanks to more reability, efficiency of the last user friendly smart instruments.
The digital pH meter has reached high levels of accuracy and repeatability, replacing in everyday analysis trend the colorimetric assays in spectrophotometry, the safe, standard, trusty metrology, but much more laborious, time-consuming, expensive and expertise technology.
Acidophilic or basophilic
The theoretical subdivision of the plants world in two categories, according to adapting acidic environments rather than basic or alkaline, has provided a third middlre class range more large, around the neutrality area, including the majority of the type, without problems of adaptability so then of survival.
Soil classification based on pH determination:
| Gessa & Testini 1989 Denomination | pH range |
| Peracid | < 5.3 |
| Acid | 5.4 - 5.9 |
| Subacid | 6.0 - 6.6 |
| Neutral | 6.7 - 7.2 |
| Subalkaline | 7.3 - 8.1 |
| Alkaline | 8.2 - 8.8 |
| Peralkaline | > 8.8 |
Measuring set up
Direct measuring in the ground by insertion of dedicated pH tip-electrode for soil is, as far as possible, but little reliable, as well as detrimental to its duration, leading to errors even coarse.
Because we can trust on it, the more steady pH value is reached through the dosing of the exact aqueous solutions concentrations.The water solubility, related on a stable solvation process degree (ionic dissociation) in hydrolysis, allowing a valid pH reading test.
The best methods recomended, SSIS (Soil Survey Information Sheet) recognized, are sampling with known volumes into two types of solutions: acqueous or saline.
A1 solutions of soil / deionized water, in ratio from 1:1 to 1:2 =10cm3/10mL or /20mLwater
S1 solutions of soil / CaCl2 solvent at 0.01 M
S2 solutions of soil / KCl solvent at 1 M
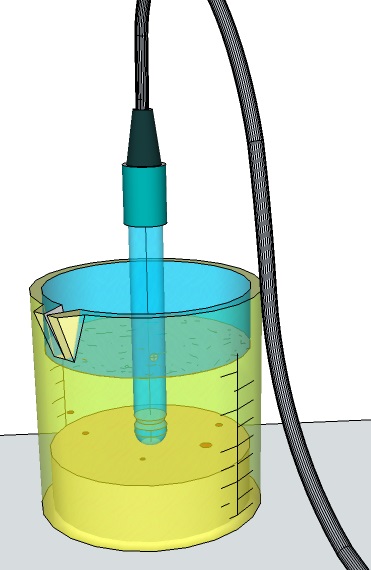
In to aqueous solution A1, the value obtained are on average higher than those of the two salt solutions (S1,S2) and different from each other, depending on whether the measurement is made with electrode diaphragm in contact on the sediment layer at bottom, in liquid dissolved, or supernatant, next to the free liquid surface.
More homogeneous and repeatable measurements are obtained with salt solutions, because more stable than the previous water solent based. The reason lies in the strong propensity of the water to the activities of ionic dissociation due to hydrolysis effect with the solute.
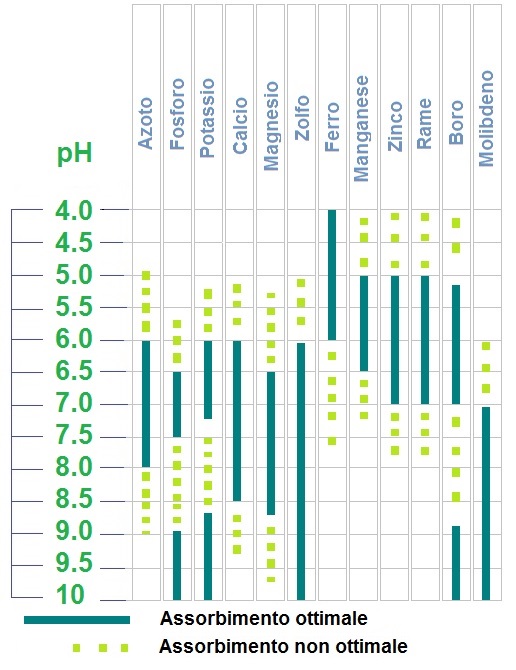
In addition, the influence of soil moisture, its oxidation, the degree of saturation reached during the solvation from the aqueous solution, represent an important variability index along the test time.
Starting from a reference value of the soil sample, dissolved in saline solution pH (CaCl2) or pH (KCl)) in relation volumes known, it is interesting to determine the subsequent relative change in the activity of hydrogen ions measured by the pH meter, or for temporal sampling systematic, or on ground with an appropriate monitoring system in line.
Example:
Add to a beaker containing 25ml of 1 M solution of KCl, 10 g (ratio 1:2.5 weight / volume) of the soil sample, dehydrated and finely comminuted with 2 mm sieve. Stir and let stand for 30' the mixture. The potentiometric determination is done by immersing the electrodes in the suspension clear. The pH value is lower than that of the acidity active.
Developments in monitoring
To run real time readings about pH changing values â€in course†on field, under the rain or irrigation water actions, due to the influence of the free activity of colloids between bases and nutrients, it becomes a valuable test case by in-depth data collection, and certainly benefits useful and meaningful informations.
Posted in: Applications support
